There is growing evidence of the importance of cannabinoids in the treatment of neurodegenerative diseases, brain injury, and ischemic stroke. In addition to their antioxidant and anti-inflammatory properties, cannabinoids also have neuroprotective characteristics and may even promote neurogenesis.
The neuroprotective effects of cannabis are mainly used in medicine to treat neurodegenerative diseases, which arise from the gradual loss of neuronal function. Parkinson’s disease is the second most common neurodegenerative disease beginning in adulthood, with Alzheimer’s disease being the most common (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1137006/). Although neurodegenerative disorders are less common in children, they do exist, usually in the form of encephalopathy.
Inflammation and the immune response are some of the main factors causing neuronal damage and dysfunction in many neurodegenerative diseases. This is why many of them occur in adulthood (https://www.ncbi.nlm.nih.gov/pubmed/20561356).
There are currently no treatments for neurodegenerative diseases. At best, drugs can only mask the symptoms and possibly slow down the further development of the disease. In this context, the neuroprotective effects of cannabis can have a significant impact on neurology (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6027455/).
Neurodegeneration and ECS
Recent research on the endocannabinoid system continues to reveal its role in neuroprotection and neurodegenerative diseases. This complex physiolytic signaling system, which is mainly concentrated in the brain, works in reverse and is one of the most interesting human mechanisms.
Unlike neurotransmitters, endogenous cannabinoids are not stored in presynaptic neurons but are produced on demand when intracellular synthesis is activated by calcium ions. This may explain why the endocannabinoid system behaves as a “recovery” mechanism (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3165950/).
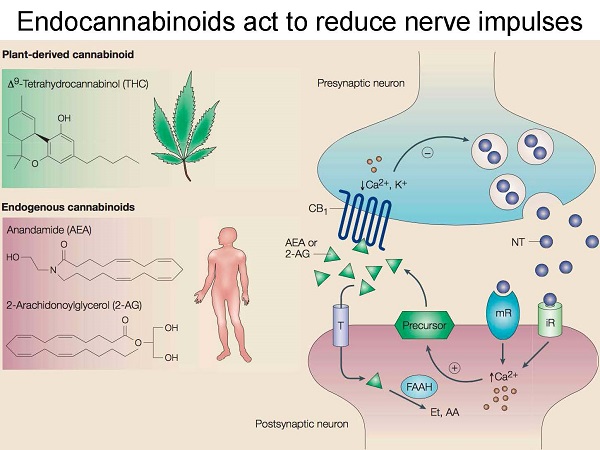
An interesting example of this is endocannabinoid levels after brain injury. A 2006 review showed that endogenous cannabinoid levels increased significantly after kainic acid-induced seizures, glutamate toxicity, shock stress, and trauma. This suggests that the endocannabinoid system is one of the main compensatory mechanisms of the brain after brain damage plays signals by endocannabinoids (https://www.ncbi.nlm.nih.gov/pubmed/16548785/).
These results suggest a possible link between the endocannabinoid system and recovery from neurodegeneration. Cannabis could be a potential target for medicine in the treatment of neurodegenerative diseases and brain injuries due to its neuroprotective properties.
Antioxidant properties of cannabis
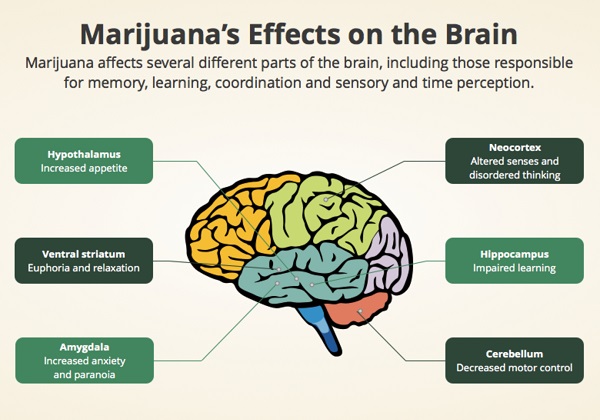
The antioxidant and anti-inflammatory properties of a variety of cannabinoids have recently become widely recognized, and our awareness of the role they play in regulating neurotransmission has deepened.
Thus, their potential as neuroprotective agents is now attracting serious attention. Studies have shown that cannabinoids can prevent neuronal death in acute injuries such as ischemic stroke and traumatic brain injury, as well as alleviate symptoms in multiple sclerosis, Huntington’s disease and other chronic neurodegenerative diseases (https://www.ncbi.nlm.nih.gov/pubmed/9653176).
Antioxidant effects are thought to be the mechanism by which cannabis can alleviate symptoms associated with neurodegenerative conditions. As early as 1998, scientists demonstrated the antioxidant effects of CBD, noting that they were stronger than ascorbate or tocopherol. Moreover, CBD does not have the toxicity associated with tocopherol (https://www.ncbi.nlm.nih.gov/pubmed/24189435/).
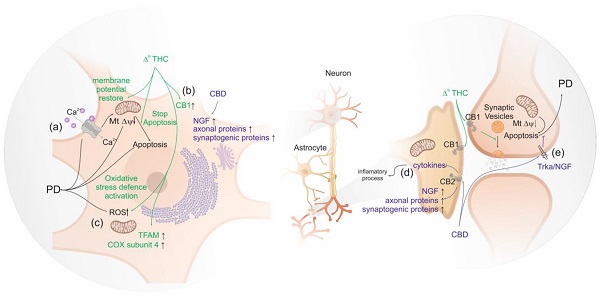
This is relevant because the brain is one of the most susceptible organs to oxidative stress. Oxidative stress is an imbalance of redox processes leading to excessive production of reactive oxygen species. It has been suggested that oxidative stress plays a key role in the development of Alzheimer’s disease. A similar assumption has been made for Parkinson’s disease (https://www.ncbi.nlm.nih.gov/pubmed/17017944).
The anti-inflammatory characteristics of cannabis can also be seen as a neuroprotective property of the plant. CB2 receptor-mediated activity on microglial cells is thought to reduce neuroinflammatory mechanisms. In the current practice of treating neurodegenerative conditions, anti-inflammatory drugs are sometimes used, especially in the treatment of Alzheimer’s disease. However, the results of studies on the effectiveness of anti-inflammatory drugs for this purpose are still contradictory.
Blocking damaging molecules
A 2002 study by Mechoulam and colleagues showed that levels of anandamide and 2-AG in the brain increase after brain injury. During such trauma, certain substances known to cause neuronal damage are produced: tumor necrosis factor-a and reactive oxygen species (https://www.sciencedirect.com/science/article/abs/pii/S1471491402022761). A study found that the synthesis of anandamide and 2-AG suppresses the formation of these potentially dangerous substances. Anandamide and 2-AG are endogenous cannabinoids produced by the body’s own endocannabinoid system (https://www.sciencedirect.com/science/article/abs/pii/S1471491402022761).
In another experiment, Mechoulam investigated the suppressive potential of 2-AG and anandamide in vivo on mice and rats with craniocerebral injuries. When both compounds were administered to animals with brain injuries, a reduction in the degree of damage was observed. There was a marked reduction in brain edema, infarct volume and hippocampal cell death as well as improvement in overall clinical condition (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5062061/).
This is relevant to medical cannabis because CBD is known to increase anandamide levels in the blood. Anandamide is cleaved by the fatty acid amide hydrolase (FAAH) enzyme, an integral membrane enzyme. Anandamide is delivered to FAAH by the fatty acid binding protein (FABP). FABP is responsible for the transport of other molecules. CBD suppresses FABP, which in turn impairs the delivery of anandamide to FAAH, increasing its level in the blood (https://www.ncbi.nlm.nih.gov/pubmed/21175579). Interestingly, the researchers also found that noncannabinoid cannabis compounds weakly inhibit MAGL, the enzyme responsible for the cleavage of 2-AG.
Cannabis and stroke
THC, CBD, and various other cannabinoids have been studied repeatedly to evaluate their neuroprotective effects during and after ischemic stroke (IS). Although there are various studies showing that cannabis use can increase the risk of stroke, it appears to be limited to a small subgroup of susceptible individuals. In 27% of these cases, reversible cerebral vasoconstriction caused by cannabinoid use may be a convincing mechanism of stroke. For most, cannabinoid therapy has great potential to mitigate inflammation and oxidative stress induced by AI (https://www.ncbi.nlm.nih.gov/pubmed/28237318).
CBD has been the subject of numerous studies as a neuroprotective agent in AI. It has been shown to increase cerebral blood flow after AI and thereby contribute to a reduction in infarct volume. 14-day repeated treatment with CBD (10 mg/kg) resulted in tolerance to hypothermia and tolerance to neuroprotective effects. For this reason, it is considered to have greater therapeutic potential than THC in this area of research. CBD has also been shown to reduce inflammation caused by the release of interleukin-1, nitric oxide, and tumor necrosis factor-a after AI (https://www.mdpi.com/1424-8247/3/7/2197).
As in CHMT, much of the damage after AI is associated with oxidative stress caused by the accumulation of AFC due to excessive glutamatergic signaling. Both THC and CBD have been shown to be effective antioxidants (as previously demonstrated) that inhibit glutamatergic signal transduction and thereby reduce the degree of AFC accumulation after ischemic stroke. Nevertheless, CBD again demonstrates greater efficacy as an antioxidant and therefore has greater therapeutic potential than THC for degenerative conditions.
Glutamate and cannabinoids
Glutamate is the most abundant neurotransmitter in the human brain. It is an excitatory neurotransmitter that participates in neuronal networks responsible for synaptic plasticity (https://www.ncbi.nlm.nih.gov/books/NBK10807/). By regulating the transmission of signals between neurons, glutamate plays a critical role in learning and memory processes. It is perhaps the most important neurotransmitter in the brain, determining a person’s ability to remember information and influencing long-term potentiality (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4679930/).
Elevated levels of glutamate can cause neurotoxicity (especially through NMDA, AMPA and kainate-glutamate receptors) and lead to the formation of harmful compounds such as reactive oxygen species (ROS) and tumor necrosis factor-alpha. Glutamate activity is also known to decrease in the presence of antioxidants (https://link.springer.com/article/10.1007/BF02462611?error=cookies_not_supported&code=27fb70f6-4293-44f5-8fd6-ee444e53948a). Since CBD and THC have antioxidant properties, it makes sense to study them in this area of neuroscience.
The interaction between different cannabinoids and the glutamatergic signaling system was investigated in a paper entitled “Neuroprotective Antioxidants from Marijuana,” published in 2000. In that study, CBD was shown to have the highest antioxidant properties to prevent glutamate toxicity (https://onlinelibrary.wiley.com/action/cookieAbsent).
Independent receptor mechanism
The authors of “Neuroprotective Antioxidants from Marijuana” confirmed that both THC and CBD improve neuronal cell protection and reduce neuronal cell toxicity by acting on NMDA, AMPA and kainate receptors. Interestingly, neuroprotection levels were not increased with specific canninoid receptor antagonists, indicating that the mechanism of action is independent of cannabinoid receptors (https://www.sciencedirect.com/science/article/abs/pii/S0303720708000051?via%3Dihub).
Previous studies have shown that CB2 receptors play an important role in these cellular processes. This could mean that selective agonists of this receptor type could have dual actions, providing protection to healthy nerve cells or inducing tumor cell apoptosis. However, CBD usually does not act as an agonist, so its neuroprotective potential must be realized elsewhere (https://www.ncbi.nlm.nih.gov/pubmed/27430346).
In addition, CBD has been shown to reduce hydroperoxide toxicity in neuronal cell cultures, confirming its effectiveness as an antioxidant. Although these tests were performed in vitro, preliminary in vivo studies in rats have shown that CBD is effective in all respects in cerebral ischemia.
Perinatal hypoxia
A significant cause of brain damage during the neonatal period is the perinatal hypoxic-ischemic event. In this case, the supply of oxygen and blood to the infant’s brain is interrupted due to asphyxia, which often occurs during delivery. This dangerous condition causes 15-20% of children with this diagnosis to die (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3010686/).
The condition can also cause serious neurological disorders, including epilepsy, cerebral palsy, impaired motor function and hyperactivity in another 25% of children. The developing brain is much more sensitive to hypoxic-ischemic events than the adult brain. Because it contains a high concentration of blood vessels and more water, the risk of damaging events such as bleeding increases (https://www.ncbi.nlm.nih.gov/pubmed/11754516).
Immediately after hypoxic-ischemic brain injury, a number of specific cellular mechanisms are activated, including increased glutamate production, which causes cellular damage and ultimately leads to excitotoxicity (a type of cell death caused by excessive transmission of glutamatergic signals).
The exact nature of this process is not yet fully understood due to the complexity of the molecular mechanisms underlying this condition. Consequently, there is a shortage of effective therapies to reduce the degree of neuronal damage. However, the current rapid progress in the understanding of this field leads to a new class of neuroprotective therapies to be evaluated and applied if proven effective.
In particular, the endocannabinoid system is being studied to determine its specific role in providing neuroprotection to the developing brain. Although the use of cannabinoids in children continues to be controversial, enough progress has been made in the use of cannabinoids for a variety of childhood conditions (including epilepsy and cancer) without harmful side effects. As a result, the controversy is now rapidly being replaced by agreement among the scientific community that such therapies may be applicable (https://www.ncbi.nlm.nih.gov/pubmed/17880390).
Cannabinoids appear to be promising candidates for therapy of perinatal brain damage. Studies have shown that they not only regulate neural responses, but also influence vasodilation through endothelial cell function and endothelin activity, control calcium homeostasis, and have significant anti-excitotoxic and anti-inflammatory effects.
There is also evidence indicating a beneficial effect of cannabinoids on the white matter of the brain, which consists of nerve fibers. In one study, introduction of the selective CB1R agonist ACEA early postnatally (from P1 to P14) promoted increased oligodendrocyte precursor formation. In the same study, WIN, another synthetic cannabinoid, stimulated myelination of subcortical white matter (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4061885/).
Studies also point to the potential of endocannabinoids as an endogenous neuronal repair mechanism. CB receptor agonists, such as THC, can activate this endogenous process.
How cannabis was used for neuroprotection
This article focuses on the phytocannabinoids THC and CBD, but also considers the case of cannabinoids in the acidic form (THC-A and CBD-A). These substances are precursors of THC and CBD and exist before a decarboxylation process that removes the carboxyl group and turns THC-A into THC (and CBD-A into CBD).
In a 2017 study published in the British Journal of Pharmacology, scientists examined the binding potential of six different phytocannabinoids to PPARγ. PPARγ is a type of nuclear transcription factor and is often used to treat diabetes because it regulates the insulin response in fat cells. However, PPARγ activation also affects microglia, which has sparked interest in its use in chronic neuroinflammatory diseases (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4882496/).
The study found that cannabinoid acids bind to and activate PPARγ much better than their decarboxylated counterparts. THC-A was found to have neuroprotective properties in mice, improving motor impairment and preventing striatal degeneration.
The sour form of THC has no psychoactive effects, making it a particularly suitable medicinal cannabinoid for research. Its lack of psychoactivity makes it suitable for a wide range of people, including children and the elderly, who are most susceptible to neurodegenerative conditions.
Conclusion
There is ample evidence in animal models of the efficacy of a variety of phytocannabinoids in treating and mitigating the effects of brain injury, ischemic stroke, and age-related cognitive decline. In addition, our understanding of the role of the endogenous cannabinoids anandamide and 2-AG in modulating inflammation and cerebral blood flow is growing. This line of research makes the endocannabinoid system a target for the treatment of neurodegenerative conditions.
Although much more research is needed, especially in humans, the amount of evidence supporting cannabinoids for the treatment of neurodegenerative disorders continues to grow. The mechanisms of action by which cannabinoids exhibit neuroprotective properties are widespread, complex, and worthy of further investigation.