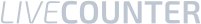Terpenes and terpenoids are aromatic compounds found in thousands of plant species and are responsible for the different flavors and aromas of cannabis. For years we have known about the presence of these compounds in cannabis, but only recently have we become aware of their potential therapeutic properties.
Terpenes are a class of natural organic compounds that include many different compounds. Terpenes and terpenoids are very similar, and the terms are often used interchangeably.
Terpenes are major organic hydrocarbons that can be found in many plants, including cannabis. These compounds are often referred to as essential oils, which are derived from the sticky resin glands of the flower. These oils are responsible for the aroma and flavor of cannabis that we know and love.
Terpenoids, also known as isoprenoids, contain more chemical elements (atoms) that have undergone oxidation. Essentially, terpenes are the wet version. Terpenoids are what terpenes become after cannabis has been dried and cured.
Isoprene rule and terpenes
Limonene is a compound consisting of two bonded isoprene units, which can be written (C5H8)2, which corresponds to C10H16. Several other terpenes also have the same structure, but the two isoprene units are arranged differently – together they form the monoterpene class.
Terpenoids consisting of three bonded isoprene links are known as sesquiterpenes (the prefix “sesqui-” means 1.5). Those containing four links are called diterpenes, and so on. The formula (C5H8)n, where n is the number of linked isoprene links, is known as the isoprene rule and is one of the most common building blocks in nature.
The cannabis plant produces cannabinoids through a complex series of chemical reactions in which the terpenes are believed to act as “building blocks”. Cannabinoids are known as terpene-phenolic compounds because they consist of terpene blocks attached to phenolic (C6H6O) groups. Given that terpenes and cannabinoids share the same precursor, the abundance of terpenes is usually a sign of high levels of cannabinoids.
The main terpenes of cannabis
Cannabis is believed to contain over 120 terpenes, although many of these are found in trace amounts and may have little, if any, effect.
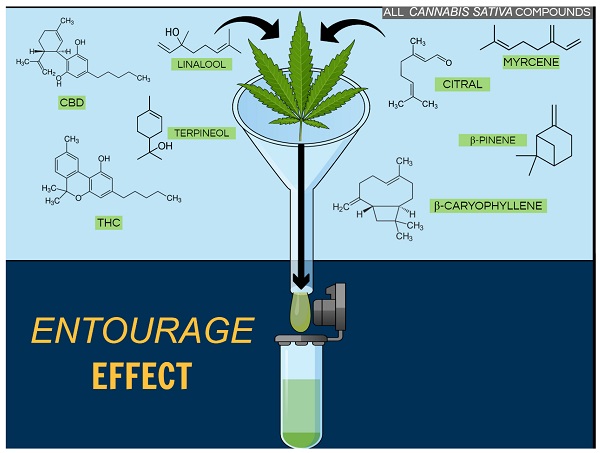
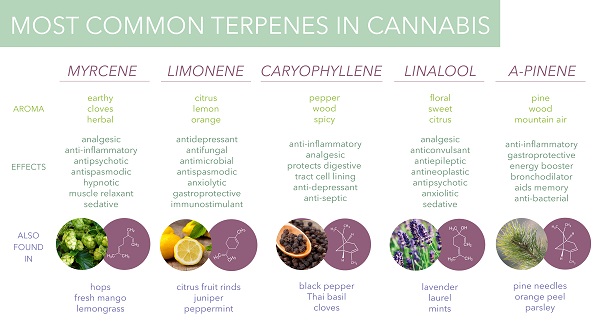
Among the major terpenes and terpenoids found in cannabis are limonene, myrcene, pinene, linalool, eulyptol, y-terpinene, ß-caryophyllene, caryophyllene oxide, nerolidol and phytol. Unlike cannabinoids (although there are now indications that other rhenium species do contain some phytocannabinoids), these compounds are not unique to cannabis, and many of them are in fact well known. The most common terpenes in cannabis are limonene, myrcene, pinene and caryophyllene.
Limonen
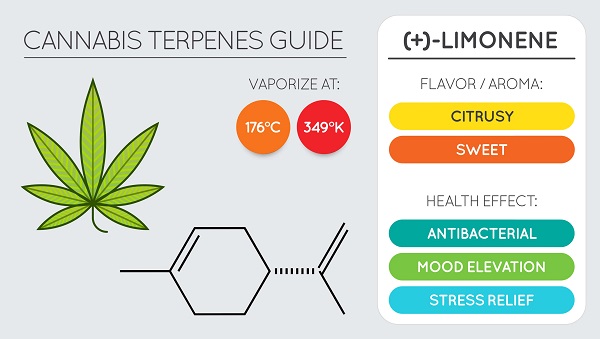
Limonene is a monoterpene that is primarily responsible for the aroma of citrus fruits, particularly the D-isomer. In its isolated form, D-limonene has a strong odor of orange and is widely used as a flavoring agent in the food industry and as a fragrance compound in perfumery.
Limonene has also found use in alternative medicine for its ability to reduce heartburn and gastric juice reflux.
Studies have shown that limonene can be effective for both chemoprevention and cancer chemotherapy. One study demonstrates its effect on the treatment of breast, lung and stomach cancer in rodents.
A recent study (2018) confirmed that limonene can promote autophagy by inducing lung cancer cell death in mice (https://www.ncbi.nlm.nih.gov/pubmed/18072821).
In addition, limonene is used as a natural renewable solvent in cleaning products due to its ability to dissolve oils (https://academic.oup.com/jn/article/129/3/775S/4722181). Under the right conditions, it can even dissolve oil in about half an hour and is considered an effective substitute for turpentine. However, people should handle limonene with caution because in high concentrations it can act as an irritant (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5894671/).
D-Limonene is also added to cannabis extracts as a flavor enhancer, as many of the existing terpenes are lost in processing (https://www.fs.fed.us/t-d/pubs/pdfpubs/pdf06732319/pdf06732319dpi72.pdf).
Mirzen
Myrcene is one of the monoterpenes and the most common terpene found in cannabis, making up over 60% of the essential oil of some strains. It is also found in bay leaf, wild thyme, hops, ylang-ylang, lemongrass, and verbena (https://books.google.com/books?id=u3zcDQAAQBAJ&pg=PA30&lpg=PA30&dq=myrcene&source=bl&ots=vUqLf5XQU1&sig=ACfU3U2V2atQdT7arThEe0f7y7egTpvytA&hl=en&sa=X&redir_esc=y#v=onepage&q=myrcene&f=false).
Myrcene is responsible for the “green hop aroma” present in dry-hopped beers. This is a beer in which hops are added after fermentation at low temperatures to enhance its “hop” flavor (https://www.researchgate.net/publication/323748761_On_the_Fate_of_b-Myrcene_during_Fermentation_-_The_Role_of_Stripping_and_Uptake_of_Hop_Oil_Components_by_Brewer’s_Yeast_in_Dry-Hopped_Wort_and_Beer). The flavor itself is described as resinous, grassy, slightly metallic and very spicy at high concentrations.
Myrcia sphaerocarpa is another plant that contains significant amounts of myrcene (https://www.sciencedirect.com/science/article/pii/S004040391300004X). It is a small shrub with astringent leaves and roots that grows in Brazil and has long been used there to treat diarrhea, diabetes and hypertension.
Myrcene has an analgesic effect in laboratory tests on rats (https://www.ncbi.nlm.nih.gov/pubmed/12587690). When used with limonene, along with the terpenoid citral, it has also been found to have sedative and motor-relaxing effects on mice.
Pinene
Pinene is another monoterpene that occurs in nature in the form of two isomers, α-inene and β-pinene (https://pubchem.ncbi.nlm.nih.gov/compound/alpha-pinene). They are usually obtained from turpentine, which is obtained by dry distillation of coniferous wood. α-pinene makes up 58-65% of the total volume of turpentine, and β-pinene about 30%.
In addition, α– and β-pinene are found in pine and other coniferous trees, as well as in sage, wormwood, and eucalyptus (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2803591/). A-pinene is also found in olives, rosemary, sassafras, and bergamot. It is the most common natural terpene. B-pinene is also found in hops and cumin.
A-pinene is known for its inhibitory effect on root growth in many plant species. This is due to the formation of reactive oxygen species that cause oxidative stress in the root system. Many plant species are thought to secrete it from their leaves as a natural herbicide, preventing other plants from competing with them for resources (https://www.ncbi.nlm.nih.gov/pubmed/21350392).
Pinene also has medical applications. In an animal study, pinene demonstrated properties against an infectious bronchitis virus. Another study showed that pinene has a bactericidal effect on methicillin-resistant Staphylococcus aureus (MRSA) and an antifungal effect on Candida albicans (https://www.mdpi.com/1420-3049/17/6/6305).
Linalool
Linalool is a monoterpenoid with the chemical formula C10H18O, which can be found in many herbs such as mint, laurel, cinnamon, birch and some citrus fruits. This chiral molecule has two enantiomers, S-linalool and R-linalool, which have different aromas and are found in different plants (https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/linalool).
S-linalool, also known as the “left” enantiomer, is found in coriander, palmarosa herb and sweet orange. Its aroma is sweet and floral. R-linalool, or the “right” enantiomer, is found in lavender, basil, and bay leaf, and has a woody and astringent aroma.
One of the medical uses of linalool is its anxiolytic action, which helps reduce anxiety (https://www.ncbi.nlm.nih.gov/pubmed/18323320). Lavender, which contains R-linalool, has been used as a sedative for many years, and recent studies on rats have confirmed its ability to modulate locomotion and motor movements.
Other interesting cannabis terpenes
-
Eucalyptol is a monoterpenoid widely found in nature. Like cannabis, it is found in eucalyptus, tea tree, bay leaf, basil and sage. It is well known for its antiseptic, antibacterial and anti-inflammatory properties.
-
G-terpinen, a monoterpene found in various citrus fruits and herbs such as oregano and marjoram, has antioxidant properties .
-
Phytol is a diterpenoid, used by insects as a deterrent to predators and also used in various household products such as detergents and soaps.
-
B-caryophyllene is a sesquiterpene found in cloves, rosemary and hops. It exhibits anti-inflammatory effects and has been shown to act as a selective CB2 receptor agonist (no other terpenes or terpenoids have been found to affect CB receptors). Caryophyllene oxide is a substance in cannabis that is identified by drug sniffing dogs .
-
Nerolidol – found in neroli, ginger and jasmine, it is a sesquiterpenoid with a fresh, woody aroma. It is currently being investigated as an aid to transdermal drug delivery (because of its ability to penetrate the skin) and as an inhibitor of the protozoan Leishmania .
-
Guaiol – is a sesquiterpenoid found in cypress and guaiacum (a genus of five slow-growing shrubs and trees native to tropical America). Guaiacum itself has been used for centuries to relieve skeletal muscle pain and upper back spasm , as well as to treat syphilis . Guaiacum is also used to increase the productivity of coughs (by liquefying mucus in the respiratory tract).
-
Eudesmol – is another sesquiterpenoid, used as a fixative in perfumery, and trans – ß – farnesene acts as a natural insecticide for many plant species, including potatoes.
Differences in terpenes depending on the strain of cannabis

The concentration of terpenes and terpenoids can vary between strains and even between related individual plants. Even two clones of the same plant grown under different environmental conditions may contain different concentrations of these compounds.
Understanding which terpenes/terpenoids a strain contains can be important because the type and concentration of each will affect the effect it causes. The major difference between the dominant terpenes in indica strains and sativa strains can be significant:
Indica
Indica strains seem to be dominated by the presence of β-myrcene, while α-pinene or limonene are present in somewhat lower concentrations. Having a high concentration of myrcene, these strains are likely to have a relaxing and sedative effect.
Sativa
The terpene profiles of sativa strains are not as reliably characterized because their profiles are not as predictable. Like indica strains, they may present β-myrcene as the dominant terpenoid, with α-pinene or α-terpinolene content as the second most important. But in some cases, α-pinene or α-terpinolenes may also be the dominant terpene. If it is the latter, the variety is likely to produce more of the typical euphoric, soaring high.
However, this is where things get even more complicated. While indica strains are known to often have high concentrations of myrcene, the researchers note that this is not always the case. They found that plants like the broad-leafed Afghan indica cannabis are more likely to contain high proportions of guaiol, eudesmol isomers and other unidentified compounds. And plants of the narrow-leaved C. indica type, which grows in the valleys of the Himalayas, contain more trans- ß-farnesene.
Conclusion
The health benefits of these terpenes are not fully understood, but they may contribute to the differences in medicinal properties found between different subspecies of cannabis. As our understanding grows, so will our knowledge of how best to develop and use new medical strains.